Thermonuclear fusion
Thermonuclear fusion is an energy source, which humans cannot use with current technologies. Although thermonuclear fusion is a rather simple nuclear reaction, to be able to provide it under the Earth’s conditions would prove extremely difficult.
Let's start from the beginning. Every atom consists of a crust and a nucleus whereby almost all the atomic weight is concentrated in the nucleus. The relationship between weight and energy is expressed by the world's most famous formula E=mc2. It means that energy and weight are principally the same - just multiplied by a constant, which by the way, is quite massive.
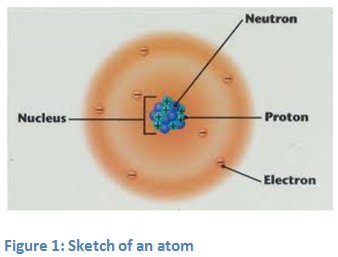
From elementary school, everyone should know that electrons in the crust are negatively charged. Positively charged protons and neutrons without charge are placed in the nucleus. The attraction between negatively charged electrons and protons in the nucleus is probably the main reason why the electrons are trapped in the atom crust. However, a much more interesting phenomenon is present in the nucleus. All the particles present in the nucleus - that is the nucleons, are extremely heavy compared to the electrons. Nucleons are both, protons and neutrons. The fact, that electrically neutral neutrons are not expelling each other in the small atomic nucleus, is not a surprise. However, it is not easy to explain why the protons do so even though all of them are positively charged and they should be repulsing one another. How then is it possible that they attract each other? The answer of this question is certainly not so simple. Therefore, it wasn’t fully explained until 1985 when a very smart man, physicist Richard Feynman shed light on the phenomenon. Although his explanation is very complex and complicated, we will simplify the story by just taking the specific part about strong interactions. Strong interaction only applies to particles, which are extremely close together, as are the nucleons in the atomic nucleus. As strong interactions are stronger than repulsive electrical interaction by a factor of approximately 100, they cause an attraction between protons and neutrons as well as between neutrons and protons. Even though electrical repulsion between protons is still present, it is far too weak to change anything. If we wanted to take a single nucleon out of the nucleus, we must overcome this strong interaction. In fact, we would have to use some energy and give it to the nucleon. This energy is called "binding energy per nucleon". Binding energy (per nucleon) is understood as negative energy, as any nucleon needs this energy to escape from the nucleus in order to become a single unbound nucleon with zero energy. Binding energy (per nucleon) is an important property of nuclei, as the stability of a nucleus is defined by this physical quantity. Light nuclei such as hydrogen isotopes and helium have a rather small binding energy. As is shown in figure 1, the binding energy curve is steep at approximately oxygen 16O . The highest binding energy has iron nucleus 56Fe, making it the most stable nucleus in the world.
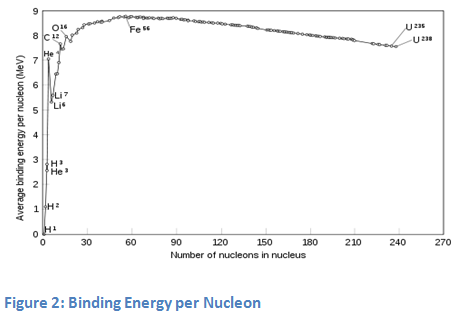
If we take two very light nuclei with small binding energies, fuse them into a single nucleus containing all the nucleons from those two very light nuclei, the binding energy per nucleon in the resulting nucleus will be higher according to figure 2. Consequentially, the energy representing the difference between binding energies would be released into the environment. As an example, let's take the fusion reaction of 2H (deuterium) and 3H (tritium) resulting in 4He. Binding energy of 4He is much higher than for both, 2H or 3H. The difference in binding energies is therefore so high, that even a nucleon (neutron) becomes completely unbounded and hence carries a huge amount of energy.
2H+3H → 4He (3,5 MeV) + n (14,1 MeV)
The biggest problem with such fusion is probably due to the conditions necessary for this reaction to occur, which are temperatures up to a few million degrees as well as high pressures. On the Earth, we would need temperatures up to 150 mil ˚C. Obviously, there is no material, which could sustain such high temperatures on Earth. Therefore, it is necessary to keep those light nuclei far away from the reactor vessel walls. This is possible through strong magnetic fields. At this point, another technical challenge becomes apparent. The magnetic fields required can only be achieved with superconductors operating at temperatures close to absolute zero. The next problem is the requirement for an abundance of tritium (3H), which is extremely rare on Earth. Therefore, 3H must be produced somewhere, most desirably from Lithium (6Li), directly in the reactor. Another technical challenge is a diverter, the only part of fusion reactors which is in direct contact with hot plasma. Finally, neutrons are the main energy carriers of the fusion reaction. Their energy is used to produce electricity. It is crucial to capture them in the right part of the reactor otherwise, they could damage and activate the surrounding materials and components of the reactor. Nowadays, the research on fusion as a future energy source is concentrated around the huge international project ITER (International Thermonuclear Energy Reactor). Further technical, interesting and scientific information about ITER and about thermonuclear fusion in general can be found on the ITER website at: www.iter.org
Michal Chudy



